

For Researchers
You can search the number of samples held by BBJ using the following criteria: sample type, gender, age at registration, name of registered disease, medical history, smoking history, alcohol consumption history, and presence of GWAS data. Please use the search results as a ” an approximate number of samples” when applying to BioBank Japan for sample distribution. Please note that the search results are current as of the time of regular data updates and may differ from the current holding status. (Japanese only)
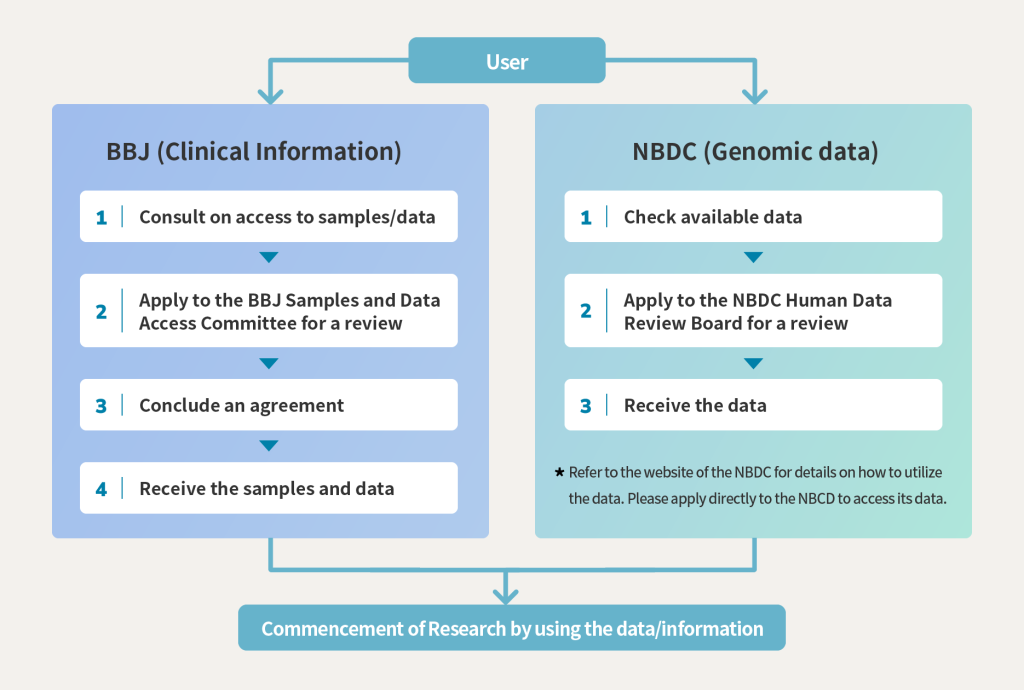
Step by Step at a glance
STEP-1 Prior confirmation / consultation on sample and data
Confirmation of the number of samples held by the BBJ using the BBJ Online Biological Samples Search System (selection of samples using clinical information)
Ethical review and approval by an ethical review committee in your institution etc.
STEP-3 Apply to BBJ /Review by the BBJ Sample and Data Access Committee
Review by the BBJ Sample and Data Access Committee for appropriateness, technical competence, research performance, etc. (Basically, electronic review)
STEP-4 Contract for distribution of material
After approval by the BBJ Sample and Data Access Committee, a contract is signed.
STEP-5 Provision of samples/data
After the contract is signed, the samples will be provided to the applicants first, followed by the data.
Receipt confirmation sent after receipt of samples Issuance of Invoicing / Payment
Completion of research, submission of report of results
*Please indicate that you used Biobank Japan’s sample and data when you write a scientific paper or make a presentation at an academic conference.
① Query Request Form of the number of available samples: Upon request, the Office of BBJ conducts an investigation to find the number of samples/data in its actual inventory. When using the BBJ Online Biological Samples Search System (Japanese only), you can download an Application Form for Investigation, and a ‘post-search inquiry number’ will be automatically assigned. Simply complete the form and return it to the Office of BBJ via email.
Contact for inquiries concerning samples and data:
The Office of BBJ
E-mail: shiryo_h“at”biobankjp.net
(Please change “at” to “@” to send e-mail.)
② Result of the investigation: You will be notified of the number of stored samples/data by email. You can consult us as to whether you should apply to the BBJ’s Sample and Data Access Committee for the samples/data, or try the search investigation again.
For genome data provided by BBJ, please confirm the following website before applying.
Available Genomic and Omics Data
For genomic and omics data that are available and shared by public databases such as NBDC, please refer to the Application Process for Obtaining Genomic/Omics data available at public database and corresponding clinical information available at BBJ (bottom of this page) and submit your application to the respective database.
Please obtain approval from the ethical review committee at your institution etc. for your research plan to use BBJ samples and data.
(1) Application to the BBJ Sample and Data Access Committee:
The Office of BBJ will send a set of application documents (electronic documents) to those research projects that have completed (or been on the process) STEP-1 and STEP-2. After completing, please upload the documents to the designated folder.
Necessary documents at the time of application:
※Please read through the following items before making an application (Japanese only):
Sample and Data Access Committee Review Criteria
BBJ Sample and Data Access Guidelines
BBJ Data Handling Security Guidelines (for Users)
Details on the process of review and guidelines for samples and data use can be found here.
(2)Electronic Review:The review process will be on-line with the exception of reviews that require lengthy discussion due to the research’s complicated nature. The decision of the review will be notified via email.
(3)Reporting of the review result: The review result will be reported to you via email.
(1)Application for an agreement: You should send an Application Form for Conclusion of Material Transfer Agreement (MTA) to the Office of BBJ.
(2)Conclusion of an MTA: After confirming the receipt of your Application Form for Conclusion of MTA, the Office of BBJ will send the contract document to you. The contract is concluded when you affix a seal to it.
After the contract is signed, the samples will be provided to the applicants first, followed by the data.
Please send a receipt confirmation to the Office of BBJ upon receiving the samples and data.
Payment: When you receive the bill, you should make payment by the due date.
Annual reports: You are requested to submit an annual report of your research and a security checklist every year in August until your research is completed. In addition, the title of the research project and the name of the research leader will be made public on our website for approved research proposals.
For genomic and omics data, some are available at BBJ database and some are available at public databases such as NBDC/AGD to promote the utilization of data.
To check Available Genomic and Omics Data
For data disclosed by BBJ, please submit an application for use to BBJ. For data disclosed by public databases, please submit an application for use to each database.
For details on the items of the clinical information (basic information and disease sheet), please refer to the following page.
Details on the Clinical Information (basic information and disease sheet) (Japanese only)
Application flow for obtaining Genomic/Omics data available at public database and corresponding clinical information available at BBJ
Refer to the following webpages for overview of BBJ’s samples and data collections and required fee