
About BBJ
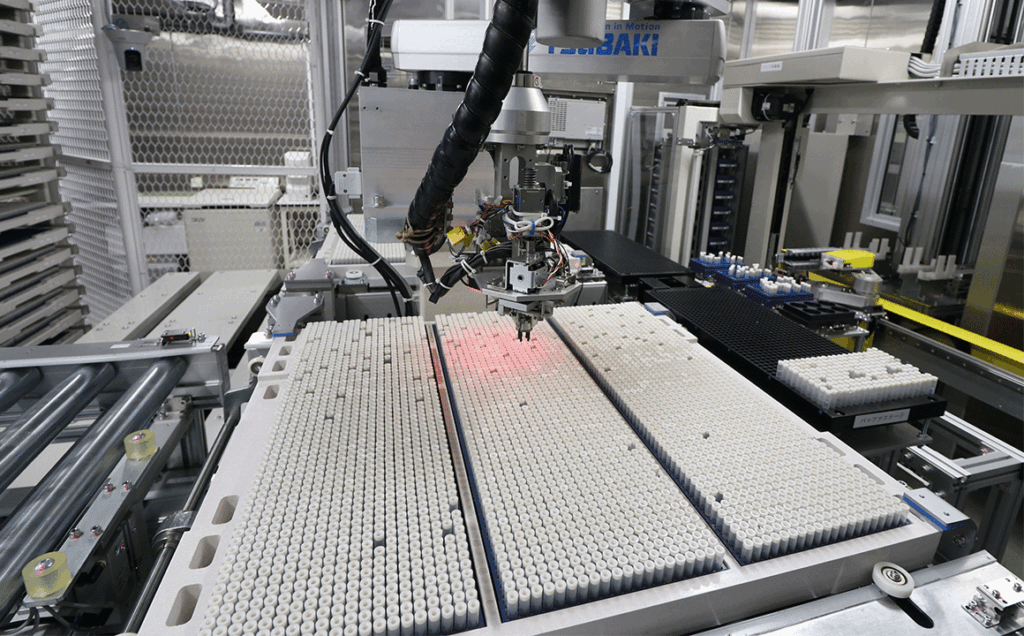
BioBank Japan stores biological samples and data, which are valuable research resources provided by participated patients, in a sample storage facilities equipped with a state-of-the-art security system under strict management in accordance with ISO international standards.
DNA bank

Serum bank

BBJ Database
The clinical information donated by the collaborating patients, including the course of illness, symptoms, and medications taken, is managed removing the name, address, date of birth, and other personally identifiable information, and assigning a new ID number.
The database of BBJ that stores the ID-assigned information is completely off-limits to the outside world and cannot be penetrated by outside parties.
Each storage unit where BioSamples are stored is equipped with its own security system, and only certain personnel are allowed to enter the room. The computer that controls the operation of the storage can also only be managed by specific personnel registered with a biometric authentication device.
※Please enjoy the video with subtitles. (No audio is included)
※Please enjoy the video with subtitles. (No audio is included)
Based on the experience and expertise on samples storage, BBJ also offers sample storage services for medical and research institutions as well as private companies. We offer two types of storage facilities: a serum storage facility and a DNA storage facility. Applications are reviewed by BBJ’s Sample and Data Access Committee. For details, please contact the Office of BBJ.
Effective October 30, 2017, the Office of BioBank Japan acquired certifications for ISO 9001: 2015 (quality management systems) and ISO/IEC 27001: 2013 (information security management systems), two international standards published by the International Standardization Organization.
This is one of our efforts to improve quality and information security in the collection, storage, and provision of biological samples and data.
For more information on samples and data held by BBJ, please refer to BBJ’s Overview of Samples and Data Collections page.


Quality and Information Security Policy (Japanese only)
Report on Handling of Biological Samples for Omics Research (Japanese only)
BBJ highly values the research resources generously contributed by participated patients, recognizing them as crucial public assets for researchers and citizens alike. As part of our commitment, BBJ regularly opens its sample storage facilities to public visitors, fostering transparency and engagement in our research endeavors.
The Institute of Medical Science of the University of Tokyo, to which BBJ belongs, accepts third-year junior high school students and high school students for sample storage tours. If you wish to visit the BBJ’s storage facilities, please apply by following the guidance on the page of the Institute of Medical Science.

Institute of Medical Science of the University of Tokyo (Japanese only)