

For Researchers
Advancing Genomic Research Through the Provision of Samples and Data to Researchers
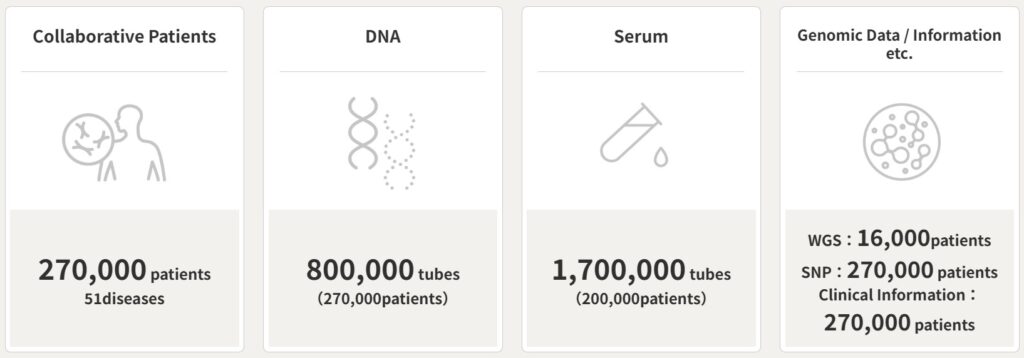
BioBank Japan (BBJ) collected DNA samples and clinical information from approximately 270,000 patients through cooperating medical institutions nationwide during fiscal years 2003-2007 and 2012-2017. Approximately 200,000 patients during fiscal years 2003-2007 also donated BBJ with serum samples. These samples and data are stored under the strictest security measures, with names and other personal information removed, and with research IDs attached. Under proper procedures and in accordance with Japanese rules (laws, guidelines, etc.), we provide those samples and data to academic research institutions, private companies, and other researchers who aim to realize genome medicine and develop new clinical and therapeutic methods.
The samples provided by BBJ originate from patients diagnosed with 51 common diseases, including lifestyle-related diseases, as of December 2023. These samples, coupled with clinical information about the diseases, constitute a highly valuable clinical and biological data for genome research targeting the Japanese population. In recent years, BBJ has intensified efforts to augment its disease-oriented biobank infrastructure by collecting additional clinical data from patients. Simultaneously, the genomic and omics analyses are being conducted using these sample and data to meet the evolving needs of researchers and fortify the biobank’s database. BBJ is committed to further enhancing its database and addressing researchers’ requirements through ongoing genomic and omics analyses. We encourage researchers to leverage BBJ’s sample and data for their studies.
Number of Diseases
51 Diseases : 267,307 patients、441,549 cases (as of July 1st, 2024)
| Disease | N |
|---|---|
| Dyslipidemia | 70,040 |
| Diabetes mellitus | 59,562 |
| Cataract | 26,012 |
| Arrhythmia | 25,354 |
| Cerebral infarction | 21,404 |
| Stable angina | 20,723 |
| Myocardial infarction | 16,637 |
| Colorectal cancer | 14,886 |
| Heart failure | 12,890 |
| Prostate cancer | 11,755 |
| Gastric cancer | 11,393 |
| Breast cancer | 11,380 |
| Bronchial asthma | 11,201 |
| Osteoporosis | 10,492 |
| Chronic hepatitis C | 8,810 |
| Lung cancer | 7,866 |
| Urolithiasis | 7,027 |
| Rheumatoid arthritis | 6,743 |
| Disease | N |
|---|---|
| Pollinosis | 6,280 |
| Uterine fibroid | 6,216 |
| Glaucoma | 6,125 |
| Unstable angina | 6,123 |
| Liver cirrhosis | 4,800 |
| COPD | 4,584 |
| ASO | 4,568 |
| Liver cancer | 4,267 |
| Periodontitis | 3,957 |
| Cerebral aneurysm | 3,941 |
| Epilepsy | 3,408 |
| Atopic dermatitis | 3,402 |
| Hematopoietic malignancy | 2,671 |
| Chronic hepatitis B | 2,666 |
| Graves’ disease | 2,493 |
| Esophageal cancer | 2,427 |
| Endometrial cancer | 2,075 |
| Disease | N |
|---|---|
| Cervical cancer | 2,025 |
| Pulmonary fibrosis | 1,917 |
| Endometriosis | 1,907 |
| Ovarian cancer | 1,610 |
| Nephrotic syndrome | 1,179 |
| Pancreatic cancer | 1,091 |
| Pulmonary tuberculosis | 1,011 |
| Drug eruption | 972 |
| Gallbladder/Bile duct cancer | 952 |
| Keloid | 896 |
| Renal cancer | 887 |
| Dementia | 820 |
| ALS | 782 |
| Depressive disorder | 541 |
| Cerebral hemorrhage | 440 |
| Febrile seizure | 341 |
The study draws its foundation from DNA, serum and clinical information originating from a cohort comprising approximately 200,000 individuals over the period spanning from June 2003 to March 2008. (47 diseases)
Serum and clinical information have been systematically collected on cases that continued to come to the hospital since April 2008. In specific cases, causes of death have been determined by examining death certificate records during prognostic investigations.
The study draws its foundation from DNA and clinical information originating from a cohort comprising approximately 70,000 individuals over the period spanning from December 2012 to December 2017. (38 diseases: 34 diseases common to 1st cohort + 4 new diseases)
DNA quality (concentrations adjusted to 100 ng/µL when purified from blood)
Results of comprehensive genomic analysis using Illumina SNP arrays
Whole genome sequencing analysis is possible even with genomic DNA stored for more than 15 years
Metabolomics analysis data of the serum samples
This is the metabolomics analysis data of serum samples stored in the serum bank of BioBank Japan as a reference for quality when analyzing our serum samples. 10 diseases (lung cancer, gastric cancer, ovarian cancer, colorectal cancer, atopic dermatitis, bronchial asthma, pulmonary emphysema, interstitial lung disease / pulmonary fibrosis, glaucoma, and rheumatoid arthritis), 50 samples for each disease (500 samples in total) were analyzed. In addition, CE-MS (capillary electrophoresis mass spectrometry) relative value data for 101 measured metabolites for a total of 105 of these samples have been added. For each disease, the mean and variance of metabolites are shown for each gender.
Please use these data as a reference for quality when analyzing serum samples.
Small panels of serum samples (in units of 100µL) are provided as a control or for screening purposes so that more researchers can utilize our serum samples.
| Serum Sample Panel (distributed as of April 2019) | ||
|---|---|---|
| Lung cancer (small cell carcinoma) | Cerebral infarction (atherothrombotic) | Nephrotic syndrome |
| Lung cancer (adenocarcinoma) | Cerebral infarction (cardiogenic embolism) | Urolithiasis |
| Lung cancer (large cell carcinoma) | Cerebral infarction (lacunar) | Osteoporosis |
| Esophageal cancer | Cerebral aneurysm | Diabetic mellitus |
| Gastric cancer | Epilepsy | Dyslipidemia |
| Colorectal cancer | Bronchial asthma | Grave’s disease |
| Liver cancer (w/chronic hepatitis B) | Pulmonary tuberculosis | Rheumatoid arthritis |
| Liver cancer (w/chronic hepatitis C) | COPD | Pollinosis |
| Pancreatic cancer | Pulmonary fibrosis/Interstitial pneumonitis | Drug eruption |
| Gallbladder/Bile duct cancer | Myocardial infarction | Atopic dermatitis |
| Prostate cancer | Unstable angina | Keloid |
| Breast cancer | Stable angina | Uterine fibroid |
| Cervical cancer | Arrhythmia | Endometriosis |
| Endometrial cancer | Heart failure | Febrile seizure |
| Ovarian cancer | Arteriosclerosis obliterans (ASO) | Glaucoma |
| Multiple myeloma | Chronic hepatitis B | Cataract |
| Hodgkin’s lymphoma | Chronic hepatitis C | Periodontitis |
| Non-Hodgkin’s lymphoma | Liver cirrhosis (w/chronic hepatitis B) | |
| Liver cirrhosis (w/chronic hepatitis C) | ||
For 11 types of cancers, pre- and post-onset serum panels (pre- and post-onset pairs) can be provided.
| Name of disease | |||
|---|---|---|---|
| Lung cancer | Colorectal cancer | Gallbladder/Bile duct cancer | Cervical cancer |
| Esophageal cancer | Liver cancer | Prostate cancer | Ovarian cancer |
| Gastric cancer | Pancreatic cancer | Breast cancer | |
■Clinical information provided for the serum panel (55 diseases)
※There are missing values. Please contact the Sample and Data Inquiry Section of the Office of the BBJ for assistance in making use of this information.
BBJ began its fifth phase of operations in a fiscal year 2023. In recent years, genomic and omics analysis methods have been rapidly developed with advances in next-generation sequencing and other analytical technologies. As a result, Genomic and Omics analysis data are widely used for genome research including biomarker discovery research and other researches aimed at developing new clinical and therapeutic methods. Against this backdrop, BBJ is promoting genomic and omics analysis of biological samples collected from the patients for further enhancing its data base for genomic medical research with the aim of implementing genomic medicine.
Genomic and omics data are available on BBJ database and also on public databases such as the NBDC human database by DBCLS as well as the Genome Group Sharing Database (AGD) in order to promote the utilization of data.
Available Genomic and Omics Data
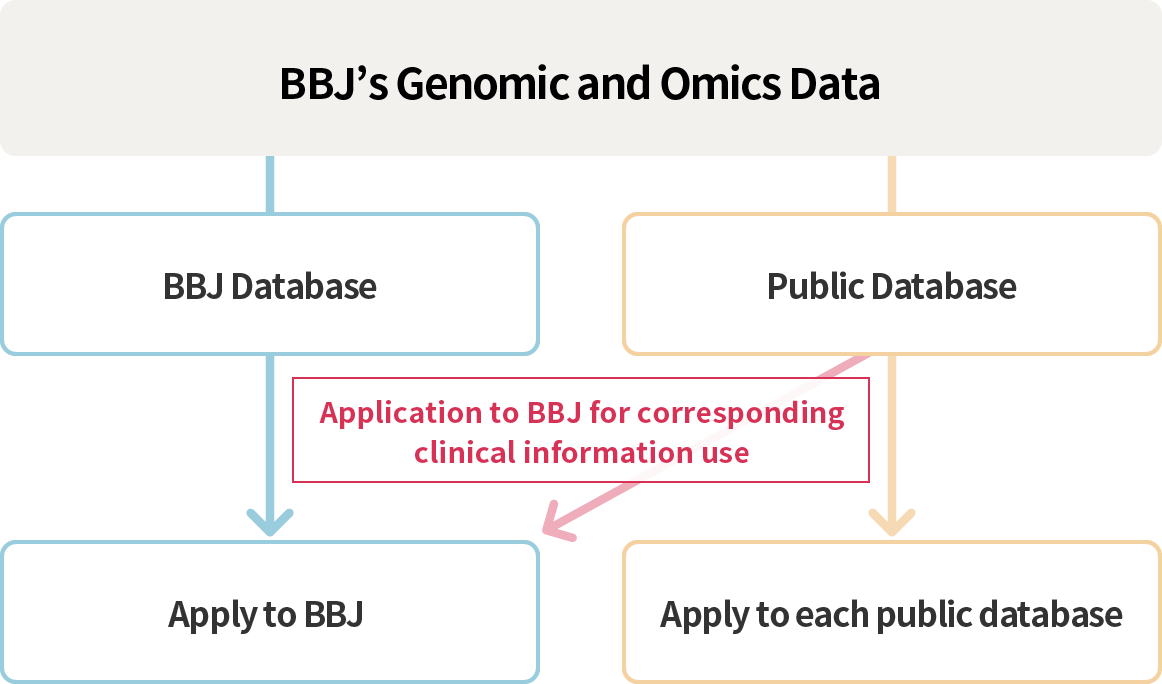
For data being available at BBJ database, please apply for use to BBJ. For data available at public databases, please apply for use to the respective database.
For clinical information corresponding to the data you have applied to use from a public database, please refer to the Application Process for Obtaining Samples and Data from BBJ. The use of the information will become possible after application is received and approved by the BBJ Sample and Data Access Committee. For detail of the clinical information, please refer to the Details on the clinical information (basic information and disease sheet)(Japanese Only).
Application Process for Obtaining Samples and data from BBJ
Using the details on the items of the clinical information (basic information and disease sheet) held by BBJ, you can refine your search for samples.
We will provide you with the clinical information in an Excel spreadsheet approximately one month after the sample is provided.
Details on the Clinical Information (basic information and disease sheet)
Shared items for all diseases : Basic items to be entered for all diseases
| Questionnaire | Details | Registration |
|---|---|---|
| Basic information | Information at registration, smoking, eating and drinking | At the time of the initial survey |
| Common classification | Diet, exercise, medical history, family history |
Shared items for all diseases : Items to be entered for all diseases
| Questionnaire | Details | Registration |
|---|---|---|
| Current Prescription | Information on medications administered within one month of the survey date | Period of the survey, multiple registrations |
| Side effect | Side effect |
Researchers can search the number of samples held by BBJ using the following criteria: sample type, gender, age at registration, name of registered disease, medical history, smoking history, alcohol consumption history, and availability of GWAS data. Please use the search results as a “sample count guideline” when applying to BBJ for obtaining samples. Please note that the search results are current as of the time of regular data updates and may differ from the current holding status.
Effective October 30, 2017, the Office of BioBank Japan acquired certifications for ISO 9001: 2015 (quality management systems) and ISO/IEC 27001: 2013 (information security management systems), two international standards published by the International Standardization Organization.
This is one of our efforts to improve quality and information security in the collection, storage, and provision of biological samples and data.
For more information on our samples storage facilities, please refer to BioSample Storage Facilities page.


We are disclosing the following information that are frequently asked by prospective samples/data users of BioBank Japan (BBJ), based on the recommendations as follows ;
Document: “Biobank Utilization/Disclosure Items for Biobank Users_Version 1-1_20250204”
| No. | Information | BBJ |
|---|---|---|
| 1 | Contact Information | Contact form URL https://forms.gle/N9MTBRuYVLPySEqk7 |
| 2 | List of BBJ’s Samples/Data | ・Available in the BBJ’s Online Biological Samples Search System (Japanese only) https://searchweb.svc.biobankjp.org/ ・Available in the Biobank Cross Search System managed by AMED https://biobank-search.megabank.tohoku.ac.jp/v2/en/ |
| 3 | SOP | Not Disclosed |
| 4 | Time Required from Sample Collection to Processing Start | Not Disclosed |
| 5 | Process for Providing Samples and Data | URL https://biobankjp.org/en/researchers/1974 |
| 6 | Time Required from Formal Request to Completion of Provision | The time required may vary depending on the type of samples and information provided <Estimated time required from the submission of the application (after ethical review completion) to the provision of biological samples and information> ・Biological samples: Approval typically takes about two weeks after submitting your application. Once approval is granted, a contract will be concluded. Samples will then be provided in approximately two weeks at the earliest. ・Data: Typically provided about one month after the provision of biological samples. |
| 7 | Provision Records | ・Provision of samples and data https://biobankjp.org/en/initiatives/1774 ・Research outcomes utilizing the BBJ’s samples and data https://biobankjp.org/publication_en |
| 8 | Provision Scheme | Provides for both Material Transfer Agreements (MTAs) and collaborative research agreement |
| 9 | Provision to External Organizations | We have a proven track record of providing samples and data to external organizations |
| 10 | Informed Consent Regarding the Provision to Private Companies | We have obtained informed consent from the patients to use their samples and data for research conducted by researchers in private companies |
| 11 | Provision to Private companies | We have a proven track record of providing |
| 12 | Provision to Overseas | At present, individual samples and data are not provided to overseas clients. The use of samples and data is permitted only within Japan. |
| 13 | On-demand Samples Collection in Response to a Request | Not conducted |
| 14 | Ownership of Intellectual Property Rights for Deliverables (under MTA) | Belongs to the user |
Quality and Information Security Policy (Japanese only)
Report on Handling of Biological Samples for Omics Research (Japanese only)